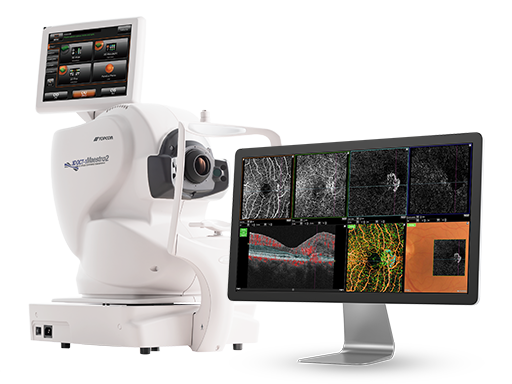
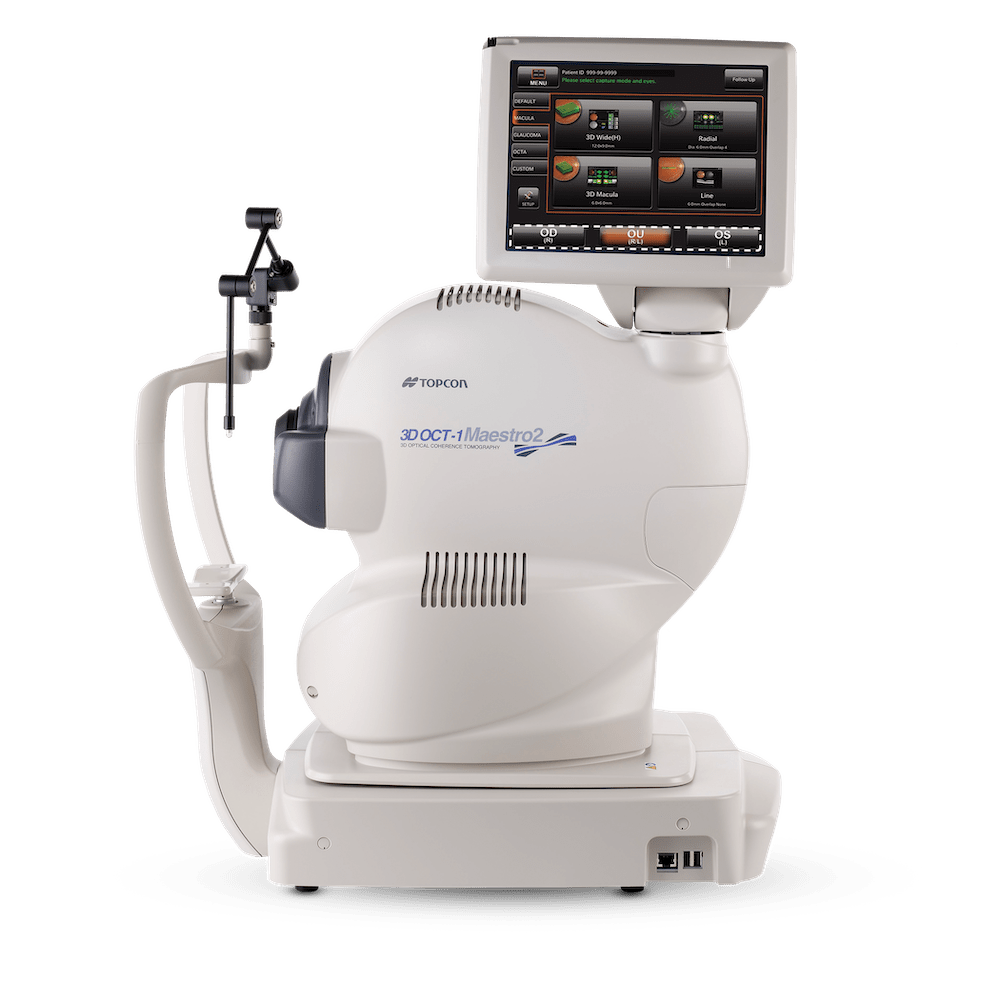
Topcon Healthcare Inc. Receives FDA 510(k) Clearance for Robotic OCT Angiography on the Maestro2 OCT Color Fundus Camera System
Maestro2 becomes the only robotic OCTA device available in the U.S.
OAKLAND, NJ – Topcon Healthcare, Inc., a leading provider of robotic medical devices and digital healthcare solutions, announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for OCTA on the robotic Maestro2, making it the first and only robotic OCT color fundus camera system with OCTA available in the U.S.
“The integration of OCTA into Maestro2 adds additional clinical horsepower to an already outstanding instrument without sacrificing ease of use,” said M. Lance Patton, chief commercial officer for Topcon Healthcare. “With its single-touch image acquisition, Maestro has been an enormous success. It is the reason Topcon has achieved the #1 market position globally for OCT devices.1 Maestro with OCTA will provide deeper insights and increase clinician confidence while continuing the workflow advantage provided by Maestro’s robotic capture technology.”
The integration of OCTA into Maestro2 adds additional clinical horsepower to an already outstanding instrument without sacrificing ease of use.
M. Lance Patton, Chief Commercial Officer, Topcon Healthcare, Inc.
The Maestro2 offers 3×3, 4.5×4.5, and 6x6mm OCTA scans, which provide the flexibility to leverage high-resolution images in the macula when assessing AMD, as well as examination of wider areas needed for diabetic retinopathy and vein/artery occlusions.
Additional features include:
- PinPoint Registration™ allows the clinician to compare subclinical OCT and OCTA findings with corresponding areas on the true color fundus photograph
- OCTA Eye Tracking improves image quality and reduces motion artifact
- Enface OCTA imaging and Angio B color-coding facilitate a better understanding of of normal and abnormal retinal and choroidal vasculature
Due to the valuable clinical information it provides and the automated capture, we anticipate that most new Maestro purchases will include the OCTA option. Doctors can also purchase OCTA upgrades for most existing Maestro2 devices.
For more information on the Maestro2 with OCTA, contact your local Topcon Healthcare representative or visit topconhealthcare.com/maestro2.
About Topcon Healthcare, Inc.
Topcon Healthcare, Inc. is part of Topcon Corporation (TSE 7732). We are a leading provider of eyecare solutions whose vision is to improve access and quality of healthcare while decreasing the cost of care. To achieve this vision, we created Healthcare from the Eye™, the strategy of applying AI models to imaging data from the eye to facilitate earlier detection and better management of disease. Healthcare from the Eye™ is powered by Harmony®, a cloud-based, vendor-inclusive digital health information platform enabling a connected care ecosystem.
A truly global business, Topcon Corporation is focused on developing solutions to solve societal challenges in the megadomains of healthcare, agriculture, and infrastructure. In healthcare, these challenges include increasing eye disease, rising medical costs, physician shortages, and unequal access to healthcare. By investing in value-driven innovations, Topcon Corporation works to help people enjoy good health and a better quality of life.
MEDIA CONTACTS
Topcon Healthcare
Leslie Amodei
VP, Marketing for Americas
[email protected]